Oak Wilt and Its Control
April 2000
Oak wilt, caused by the fungus Ceratocystis fagacearum, is found throughout Illinois. The disease continues to kill oaks in the state every year in residential areas, parks, farm woodlots, and forests. In mixed stands of white and red oaks, red oaks may die out leaving a pure stand of white oaks.

Figure 1. Symptoms of
Oak Wilt on Red Oak
The oak wilt fungus invades the water conducting vessels of the sapwood through fresh wounds or by root grafts formed between diseased and healthy trees. In a few days, balloon-like tyloses and gums begin to plug the water conducting tissue, blocking the flow of water and nutrients from the roots to the foliage. As the supply of water becomes restricted, leaves wilt and die (Figure 1). No complete control or cure for oak wilt exists. However, proper care plus mechanical and chemical control measures can keep the disease from spreading to healthy trees nearby.
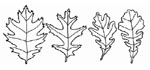
Figure 2. Oak Groups:
Red, Black, and Pin Oak
Have Pointed Tips;
White and Bur Oak
Have Curved Lobes
Without Pointed Tips
Symptoms
RED-BLACK OAK GROUP (leaf lobes pointed): includes black, black jack, pin, red, scarlet, and shingle oak (Figure 2). The leaves in the top of an infected tree and the tips of the lateral branches discolor and wilt in late spring and early summer. The wilt symptoms progress downward and inward until all the foliage is affected. The leaves curl slightly and turn a dull pale-green, bronze, or tan starting at the margins. Defoliation may occur any time after the symptoms appear. By late summer, an infected tree is often bare of leaves.

Figure 3. Branches of
Red Oak with Bark
Removed Showing
Healthy Sapwood (top)
and Streaked Wood
(middle and bottom)
Typical of Infection by
the Oak Wilt Fungus
Mature leaves usually remain stiff during the different stages of wilt and for some time after the tree dies. Immature leaves curl, droop, turn dark brown to black, and remain on the branches. A brown or black discoloration usually develops in the current-season sapwood of wilting branches (Figure 3). The discoloration may appear as longitudinal streaks. In cross-section, a brown ring or circle of dark-colored spots is evident. Oak wilt is the primary suspect when clusters of red and black oaks start dying. They often wilt completely within 4 to 6 weeks after the first symptoms appear or during a single growing season. Once infected, oaks in the red and black group do not recover.
Occasionally, large branches of trees infected late in the summer survive the winter but die the following spring after producing a few scattered leaves. Oak wilt is the primary suspect when localized areas of dead and dying red and black oaks showing the described symptoms continue to increase.
WHITE-BUR OAK GROUP (leaves with rounded lobes): includes bur, swamp, white, and chinquapin oak (Figure 2). The leaves on affected branches usually become light brown or straw-colored from the leaf tip toward the base. The leaves curl and remain attached to the branches. Infection generally occurs in scattered branches of the crown. The trees may die in one year, but usually die slowly over a period of several years or more. After two or more years of progressive die-back, infected white oaks have sparse crowns and eventually die from oak wilt or secondary causes. Bur oaks are intermediate in susceptibility and may be killed as quickly as red and black oaks or as slowly as white oaks. Sapwood discoloration, similar to that in red and black oaks, has occasionally been observed in wilting bur oak trees. White oaks have been known to recover after one season of infection when new wood is laid down over an infected annual ring effectively “walling off” the fungus and allowing tree recovery. This is usually only a temporary recovery.
Oak wilt can be confused with other problems such as anthracnose, construction damage (including soil compaction), changes in the soil grade or water table, lightning damage, nutritional disorders, insect and animal injuries, chemical damage and root decay. (Anthracnose commonly affects the leaves on the lower branches of white oaks; oak wilt usually affects the upper part of the tree first).
Laboratory Culturing
Some communities use trained survey people to locate and identify diseased trees by visual symptoms. Positive identification of oak wilt is possible by laboratory culturing (especially from late May to mid-July) and recovering the oak wilt fungus from the tree. For laboratory confirmation, send two to four branch sections from live, freshly wilting branches. Branches that are dead and dried out (where the bark can not be easily peeled away) should not be submitted. The sections should be about thumb thickness and 6 to 8 inches long. The fresh branch sections should be sealed in a plastic bag attached to a completed Plant Clinic specimen data form. These forms are available at University of Illinois Extension offices, and online at the University of Illinois Plant Clinic. Do not add moisture to the branch sections, but do protect them from excessive heat and from drying out. Some southern states recently reported increased success in culturing the fungus when samples were kept cool during shipping. The University of Illinois Plant Clinic has experienced similar results. We highly recommend mailing samples early in the week and shipping them on ice. For example, you might include a small, plastic soda bottle, partially filled with frozen water along with the sample.
The fresh branch sections should be mailed to the Plant Clinic, 1401 W. St. Mary's Road, Urbana, IL 61802. There is a charge per sample for culturing – call ahead (217-333-0519) for current prices. Include a check, payable to the University of Illinois, with the oak-branch sections. The results of the laboratory diagnosis will be mailed as soon as they are known. The process usually takes one to two weeks of lab time.
Hosts
The known hosts of the oak wilt fungus include 36 species of oaks (Quercus species) as well as the closely related American chestnut (Castanea dentata), Chinese chestnut (C. mollissima), Spanish chestnut (C. sativa), Allegheny chinquapin (C. pumila), bush chinquapin (Castanopsis sempervirens), and tanbark-oak (Lithocarpus densiflorus). All species and varieties of oak tested to date have been found susceptible to the oak wilt fungus.
Spread
Oak wilt moves from diseased to healthy trees in two ways – through root grafts formed between trees and through fresh wounds via sap-feeding insects.
Several species of sap-feeding beetles (Nitidulidae family). Beetles spread the oak wilt organism by carrying spores to fresh wounds on healthy oaks. The spores are produced on fungus mats formed beneath the bark of wilt-killed trees of the red oak group and as the beetles crawl over and feed on the fungus mats, the microscopic spores stick to their bodies. The insects then fly to healthy oak trees and feed on the sap oozing from fresh wounds. Squirrels are potential carriers of the wilt fungus, too, but their importance has not been established.
Root grafts. Where trees are growing close to one another, their root systems often become intertwined. Thus, the roots of one red oak may graft to the roots of a nearby red oak. If one of these trees becomes infected, the root grafts serve as natural “pipelines” for the oak wilt fungus to spread, below ground, to the healthy tree. Root grafts are most common between oaks of the same species. For example, it would be rare to find root grafting between a white and a red oak.
Management
Control measures are designed to keep the disease from spreading by preventing unnecessary wounds, severing root grafts either mechanically or chemically, and removing and destroying diseased trees early. In some instances, fungicides may be used where high value trees are in danger and when all other appropriate control measures have been implemented.
- Avoid unnecessary wounds. Wound infections are most likely to occur during the spring and early summer between the time the buds begin to swell and full leaf development. If possible, do not start construction on sites with oak trees during the critical period of spring and early summer. Generally it is suggested that oaks be pruned in the fall after a hard freeze. A very cautious approach, for areas known to have Oak Wilt, would be to prohibit cutting from bud swell to October. If you must prune during this period, consider treating the wounds with a wound dressing. Where oak wilt is not a threat, the use of tree wound paints and dressings is no longer recommended.
- Control root grafts. Four factors influence the likelihood of root grafting between two trees: 1) trunk diameter of both trees; 2) distance between trees; 3) soil type and drainage; and 4) tree species. For example, research shows that large (12"diameter) trees growing in sandy soil will likely form grafts if the distance between them is less than 93 feet. However, the same size trees growing in heavier, loamy sand may form root grafts if the distance between trees is less than 74 feet. In other words, root s are likely to spread farther in sandy, rather than heavy soil. While there is no similar published research based on heavier (high clay content) soils common to much of Illinois, we can make some generalities.
Oaks growing in heavy clay soils that are within 30 to 50 feet of diseased trees are likely root grafted and should be considered as "infected suspects". This means that they may already be infected but are not yet showing symptoms. As previously mentioned, root grafts are most common between oaks of the same species, so it is not necessary to place barriers between a member of the red/black oak group and a member of the white/bur oak group.
All possible root grafts between healthy and suspect or infected trees should be severed mechanically or chemically before the diseased trees are removed. These two methods are described below - choose the best method for the particular situation. You should work with a experienced forest pest specialist, forester, or consultant trained in oak wilt management to determine the location of barriers. In addition, before placing any type of barrier, it is important that you call JULIE (800-892-0123) or, in the Chicago area, DIGGER (312-744-7000). These folks will arrange to have your underground wires, pipes, etc. located and marked within 48 (business) hours after your request. While it is important that you sever root grafts as soon as possible after an oak wilt diagnosis, do it right and don't be careless.
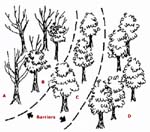
Figure 4. Typical Oak
Wilt "Pocket",
Indicating Where
Barriers Should BeWhere there is a mixture of diseased, suspect, and healthy oaks, you should make two barriers – one between the healthy and suspect trees and another between the suspect and diseased trees (Figure 4). If you plan to mechanically disrupt the roots, be on the cautious side and make the barriers in the above order. The goal is to sever all roots to a depth of 3-5 feet in a line midway between the two trees. Experience and limited research indicates t hat deeper (4-5 feet) barriers are more effective t han shallow barriers. When sidewalks, driveways, curbs, or other obstacles prevent establishing a completed barrier, extend the barrier along the obstruction and sever all root connections.
In residential areas, infected trees commonly involve more than one homeowner. For best results, all homeowners in the neighborhood must: 1) be informed; 2) understand the necessity of establishing barriers regardless of property boundary lines; and, 3) carry out a continuing neighborhood-control program.
- Cut the roots mechanically. While neither method is foolproof, it has been shown that mechanical barriers are much more effective than chemical barriers. Any trenching machine or vibratory plow (sold by Ditch Witch or Vermeer or other companies) that will cut or break the roots to a depth of 3-5 feet (see above discussion) can be used. Many practitioners prefer to use a vibratory plow because it slices through the soil and does not require backfilling with soil. Root-cutting equipment is commonly available from a local forester, commercial arborist, utility company, or irrigation installation company. However, it may be difficult to find a vibratory plow with a shank longer than 3 feet (5 foot shanks can be custom-built). The trenching technique is not suitable near sidewalks, driveways, buried pipes, power lines, or telephone cables. In those situations, chemical treatment might be necessary.
- Employ a chemical treatment. Metam sodium (sold as Metam Sodium and other brand names) will kill segments of grafted roots. Fumigants are restricted-use pesticides so they must be applied by a licensed pesticide applicator who has been trained in their use. Typically the fumigant is placed into 1-2 inch-diameter holes drilled 18-24 inches deep and spaced 4-6 inches apart. The fumigant diffuses into the soil to various degrees and kills all roots (including grass and other plants along a strip about 18 inches wide), blocking the spread of the oak wilt fungus from diseased to healthy trees. Because they are inherently dangerous, difficult to apply properly, costly, and less effective than mechanical barriers, fumigants should only be used as a last resort.
- Cut the roots mechanically. While neither method is foolproof, it has been shown that mechanical barriers are much more effective than chemical barriers. Any trenching machine or vibratory plow (sold by Ditch Witch or Vermeer or other companies) that will cut or break the roots to a depth of 3-5 feet (see above discussion) can be used. Many practitioners prefer to use a vibratory plow because it slices through the soil and does not require backfilling with soil. Root-cutting equipment is commonly available from a local forester, commercial arborist, utility company, or irrigation installation company. However, it may be difficult to find a vibratory plow with a shank longer than 3 feet (5 foot shanks can be custom-built). The trenching technique is not suitable near sidewalks, driveways, buried pipes, power lines, or telephone cables. In those situations, chemical treatment might be necessary.
- Remove dead trees. Diseased and dead oaks should be removed and burned as soon as possible after mechanical root cutting or about 2 weeks after chemical treatment for root grafts, unless removal would wound surrounding trees. If that is a possibility, remove the diseased or dead oaks in late fall or winter. Since fungal mats are not known to develop within members of the white oak group, there is less urgency in removing dead or dying trees that belong to this group. However, remember that all dead shade trees present a landscape hazard and should be removed as soon as possible after death.
If diseased white oaks are especially valuable, remove the wilting and dead branches carefully at the trunk, treat the wounds promptly, and water during periods of drought. Pruning tools should be disinfected before trimming another tree or noninfected tissue. To disinfect tools: (1) remove wood fragments; (2) soak for several minutes in a disinfectant such as 70 percent rubbing alcohol or liquid household bleach (diluted 1 to 5 with water), and then (3) rinse the tools in clean water. If wilt symptoms continue to advance beyond the pruned parts, the tree will probably die of oak wilt or secondary complications. In such cases, the white oak should be removed.
The use of diseased oaks for firewood is not recommended in residential areas since the firewood is frequently not burned before the following spring when insects may carry the wilt fungus out of the infected wood. If diseased wood is to be used, it should be processed as soon as possible and burned before spring. Firewood should be cut to the proper length, split, stacked off of the ground in a single tier, and protected from moisture in order to hasten drying. Firewood that has been debarked or stored in a dry place is not a source of infection. Diseased oak timber can be harvested for lumber if it is sawed before the following spring.
- Control infections that jump barriers. Occasionally, oak trees beyond the barriers become infected in spite of the severed roots. Additional barriers should then be put down using the same procedure to surround newly found diseased trees and any neighboring oaks that are suspect.
- Create a barrier of poisoned oaks. In timbered areas where it is not feasible to sever root grafts, use fungicides, or properly dispose of infected oaks, foresters may contain pockets of diseased oaks by using herbicides to deliberately kill diseased and nearby "suspect" and healthy oaks. This poison barrier should be set up as soon as possible after the disease is observed. For example, creating one or two barriers of poisoned red oaks around the single infected red oak or around a pocket of wilt-infected red oaks (Figure 4) will prevent the fungus from spreading through root grafts. The diseased trees should also be poisoned. Both the tops and the roots of the poisoned oaks must be killed to stop transmission by root grafts. Consult the Brush Control chapter of the Illinois Agricultural Pest Management Handbook (updated annually) for current herbicide recommendations. The poison barrier system of oak wilt management should only be performed by a appropriately licensed and trained forester or arborist, and should be considered only for oak trees having value as timber and not for high-valued trees having ornamental value.
- Use systemic fungicides for high value trees. Researchers and practitioners continue to investigate the use of systemic fungicides in an attempt to provide safe, long-lasting protection against oak wilt. While there are several injectable fungicides labeled for the control of oak wilt, many researchers and practitioners agree that Alamo is currently the most effective. While Alamo can be applied using the newer "micro-injection capsules", most practitioners prefer the traditional "macro-injection" technique. The drawback to using any of the current fungicides is cost (typically $300 or more per tree) and the potential need for re-treatment in one to two years. Thus, fungicides are suggested only where high value trees are in danger and when all other appropriate control measures, listed above, are used. Alamo is labeled for use as preventative and therapeutic injections. Therapeutic injections have the best chance of working if the tree shows less than 10-20% crown loss due to oak wilt. However, researchers and practitioners tend to agree that it is a waste of money to inject members of the red oak group that show any symptoms of Oak Wilt. Tree injections should only be made by trained arborists or others trained in injection techniques and diagnosis of Oak Wilt. Consult the Illinois Commercial Landscape and Turfgrass Pest Management Handbook (updated annually) for current chemical recommendations.
For further information on Oak Wilt or other tree diseases, contact Nancy R. Pataky, Extension Specialist and Director of the Plant Disease Clinic, Department of Crop Sciences, University of Illinois, Urbana-Champaign. We acknowledge with thanks the expertise of Bruce E. Paulsrud, Extension Specialist and Pesticide Applicator Educator, in the revision of this publication.
University of Illinois Extension provides equal opportunities in programs and employment.