Phomopsis Twig Blight of Juniper
August 1999
Phomopsis twig blight of juniper, also known as nursery blight, cedar, juniper, or needle blight, is caused by the fungus Phomopsis juniperovora. Economic damage to landscape plantings and nursery stock is largely restricted to species and cultivars of juniper (Juniperus). Other evergreens that are attacked include arborvitae (Thuja), species of true cedar (Cupressus), and false cedar (Chamaecyparis), European larch (Larix decidua), jack pine (Pinus banksiana), English yew (Taxus baccata), Japanese plum yew (Cephalotaxus drupacea), Douglas fir (Pseudotsuga taxifolia), species of fir (Abies), and Cryptomeria japonica. The disease is mainly a leaf and shoot infection found in young plants and on the new growth of older plants.

Figure 1. Phomopsis
Twig Blight on Juniper
Shoot (Note Pycnidia of
Phomopsis Fungus on
Stem)
Juniper seedlings and transplants in nurseries are highly susceptible and are commonly killed by the blight. Overhead irrigation in a nursery is conducive to infection, and a large number of seedlings, transplants, or grafts can be blighted and killed in a very short time. The value of older nursery stock diminishes markedly from infection and the survival of this stock is poor after planting. The disease be-comes progressively less serious as trees and shrubs grow older, even though the new growth of older plants is very susceptible, death or severe damage to a plant over five years old is much less likely.
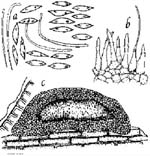
Figure 2. Phomopsis
juniperovora, the Cause
of Phomopsis Blight of
Junipers Seen Under
Microscope (Drawing L.
Gray)
During prolonged wet, warm periods in spring and summer (April through early June) and again in late August and September, the fungus becomes particularly infectious. Phomopsis twig blight to juniper is found in most of the United States–from the Pacific Northwest, throughout the Great Plains from South Dakota to Texas, and eastward through the entire Midwest to the East Coast–as well as in Canada, however, it is not common on plants growing in the wild.
Symptoms
Yellow spots at the shoot tips of young needles are the first symptoms to appear, although older needles may also show the spotting. As the infection progresses from the needles into the stems, a gradual dieback of the new shoot growth occurs changing from light yellow to red brown to ash gray, eventually killing the entire branch.
Lesions occur on the stems and frequently develop into cankers at the junction of healthy and diseased tissues. Small stems (less than 1/3 inch in diameter) are usually girdled by these cankers, causing the stem tissue beyond the cankers to die. Older branches (more than 1/3 inch in diameter) are more resistant to infection, and cankers that form on them usually heal. In advanced stages of infection, small black spots (the fungus fruiting bodies or pycnidia) can be seen with the unaided eye or with a magnifying glass on the dried, ash gray parts of stems and needles (Figure 1). The central part of a plant is often more affected than its outer portion, with the new growth showing almost continuous infection. Under certain conditions favorable for fungal development, the entire shrub or tree (especially a young plant) may be affected, and all its needles and stems will die and turn brown.
Phomopsis twig blight is easily confused with four other problems of junipers:
- Under drought conditions, the -tips of branches may be killed, but the line of demar-cation between green and dead tissues is gradual, while in Phomopsis blight, this line is sharp;
- Damage from the lesser cornstalk borer (Elasmopalpus lignosellus) can be distinguished from Phomopsis blight by the straw color of the dead tops and by the feeding wounds on the lower stem and taproot;
- Another fungus, Cercospora sequoiae, causes a blight in junipers and related species, but in this disease only the needles, not the stems (as in Phomopsis blight), are infected. Also, infection by Cercospora starts on the oldest needles of the lower branches, spreading upward and outward, whereas infection by Phomopsis starts in newly developed needles and progresses inward; and,
- A third fungus, Sclerophoma pythiophila, perfect state Sydowia polyspora, causes a tip dieback with symptoms very similar to those of Phomopsis. Sclerophoma attacks weakened or damaged juniper tips in Illinois and Wisconsin, especially front-injured tips. The only way to distinguish Phomopsis from Sclerophoma is through microscopic examination of the pycnidia. The main difference between the two fungi is in the spores that are produced within the pycnidia. Phomopsis produces two distinct types of conidia, alpha and beta (Figure 2), while Sclerophoma produces only alpha-type spores that do not have the two oil globules of Phomopsis. Other hosts besides junipers in North America that have been reported as being infected by Sclerophoma include Norway (Picea abies) and Engelmann spruces (P. Engelmannii), numerous pines (Pinus spp.), Douglas fir (Pseudotsuga menziesii), tamarack (Larix laricina), and white fir (Abies concolor). Sclerophoma is considered a weak pathogen on evergreens and associated with tissues injured by insects or unsuitable climatic conditions.
Disease Cycle
The most common sources of infection each spring are spores (conidia) produced in fungal fruiting bodies (pycnidia) that appear as black spots on the needles and stems infected the previous year (Figure 2). New pycnidia formed in the current growing season are also a source of infective spores. The microscopic spread by the wind, insects, and during pruning and other handling operations. Under moderate temperatures (60° to 82°F or 15° to 27°C) and high humidity, the spores germinate within seven hours after coming in contact with young needles, especially those still in the yellowish green stage. After the needles mature (develop a normal, deep green color), they are not susceptible to infection.
Germinated spores of Phomopsis are not killed by drying, like many other fungi, but begin growing again when moist conditions return. Within three to five days after infection, the fungus permeates the young needles and quickly invades young stem tissue. After colonizing a side shoot, the fungus mycelium progresses into the main stem, growing rapidly along the inner bark, killing the cambium and staining the wood a brownish color.
Within three to four weeks after infection, pycnidia develop on the needles and stems that have died and turned ash gray. At first, the pycnidia are embedded in the tissue, but later, after the infected tissue has dried considerably, they partially erupt through the epidermis. During wet, warm weather, spores ooze from the pycnidia and are easily and quickly dispersed. The fungus can persist as mycelium in dead parts of infected plants for as long as two years.
Cultural Control
- Plant only resistant species, varieties, and cultivars of junipers.
- Where practical, prune out and burn all blighted parts as they appear. Restrict pruning or shearing to periods of dry weather. Infection can be further reduced by restricting pruning to periods when the resulting new growth, stimulated by pruning, occurs in the drier part of the season, which is from late June through early August.
- In nursery operations:
- Every seven to ten days, in dry weather, remove and burn all infected seedlings or place them in sealed plastic bags and haul them to a sanitary landfill.
- If possible, avoid having juniper seedbeds adjacent to beds containing older junipers or other susceptible stock.
- Avoid planting in poorly drained areas.
- If overhead sprinklers must be used, irrigate seedling beds early in the day to allow for drying before nightfall.
- Avoid using shading frames because these increase the length of time that moisture remains on the foliage.
- Do not use junipers or other hosts as windbreaks or as landscape plantings in or around a nursery because they may become a source of spores for nursery stock.
- Avoid wounding trees when cultivating and transplanting them.
- Do not use branches or needles of junipers or susceptible plants for mulching.
Chemical Control
- In nurseries, apply a suggested fungicide to seedlings throughout the growing season, beginning at seedling emergence. Weekly elimination of all infected seedlings, and spraying with a fungicide every 7 to 10 days have been shown to be very effective. Fungicide recommendations are given in Illinois Urban Pest Management Handbook which is revised annually.
- For landscape plantings, fungicides give effective control when applied at the right time. Since only new growth is susceptible, spray at budbreak and then repeat at 10- to 14-day intervals until the new growth has matured (when needles have changed from light yellow green to dark green). Spray also when new flushes of growth appear in the summer and early fall, or in response to pruning or shearing. If extended periods of wet weather persist, spray every 10 to 14 days as long as young, susceptible needles are present.
- Because the fungus can only invade and attack young, tender, unwounded needles of healthy, vigorous plants, keeping new growth thoroughly protected by regular spraying is very important.
Contact Nancy R. Pataky, Extension Specialist and Director of the Plant Disease Clinic, Department of Crop Sciences, University of Illinois at Urbana, for further information on diseases of ornamental plants.
University of Illinois Extension provides equal opportunities in programs and employment.